GAO: American Nuclear Plants Vulnerable to Lithium Shortage - News - Nuclear Power News - Nuclear Street - Nuclear Power Plant News, Jobs, and Careers
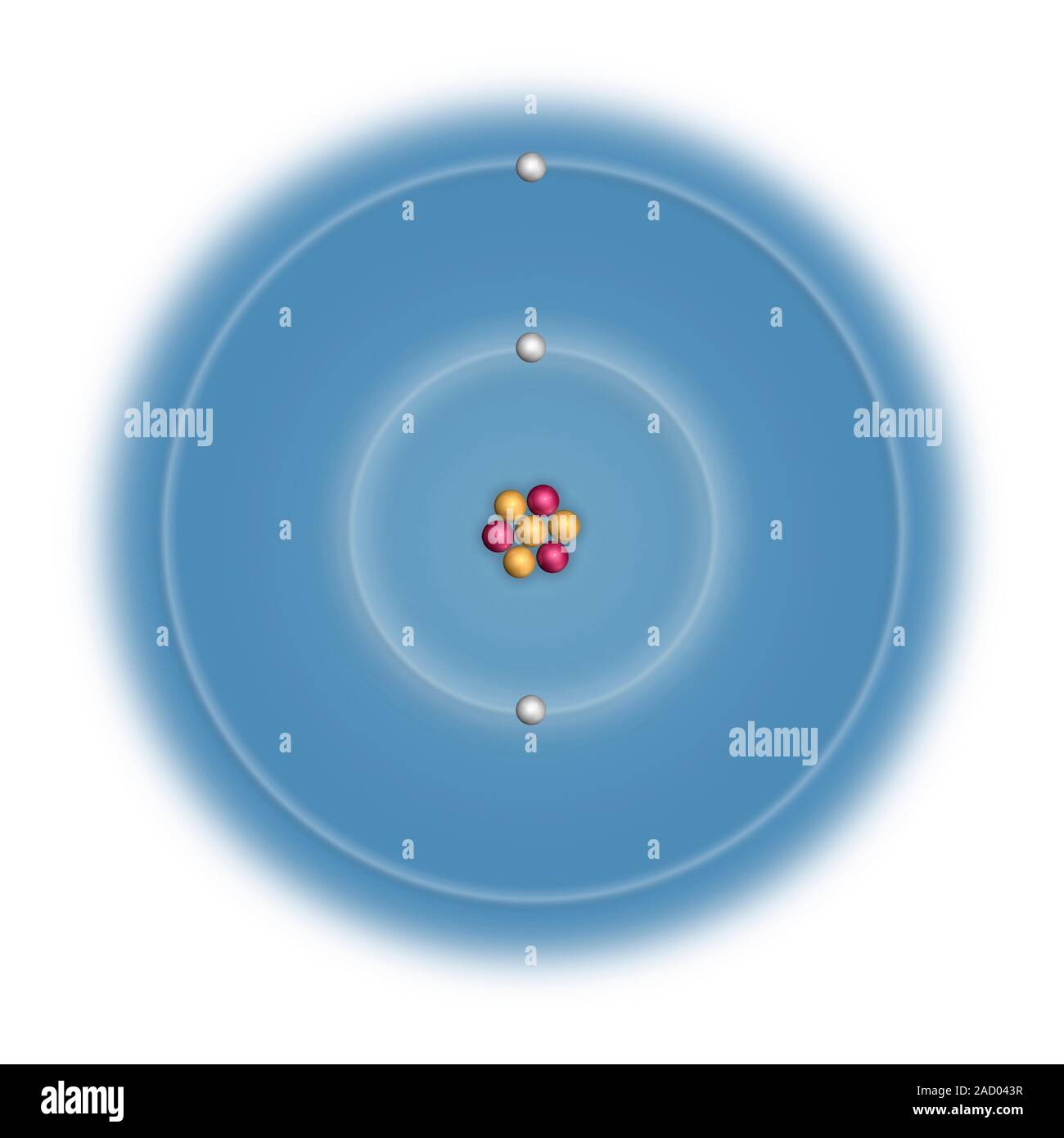
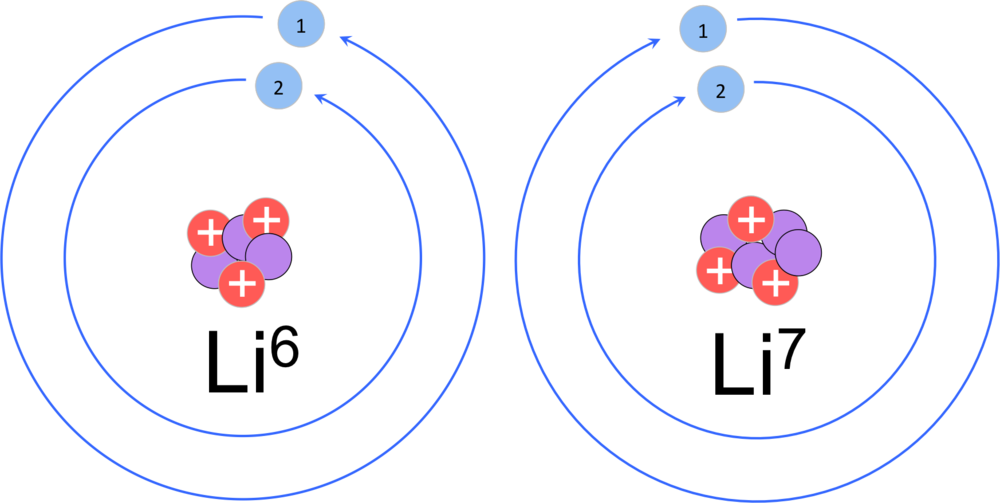
Lithium (Li). Diagramm mit der atomaren Zusammensetzung und Elektronenkonfiguration eines Atoms von Lithium-7 (Ordnungszahl: 3), die häufigste Isotop o Stockfotografie - Alamy

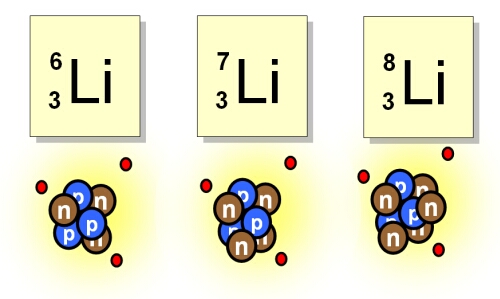
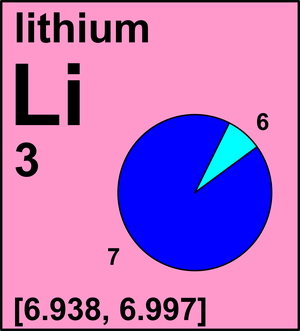
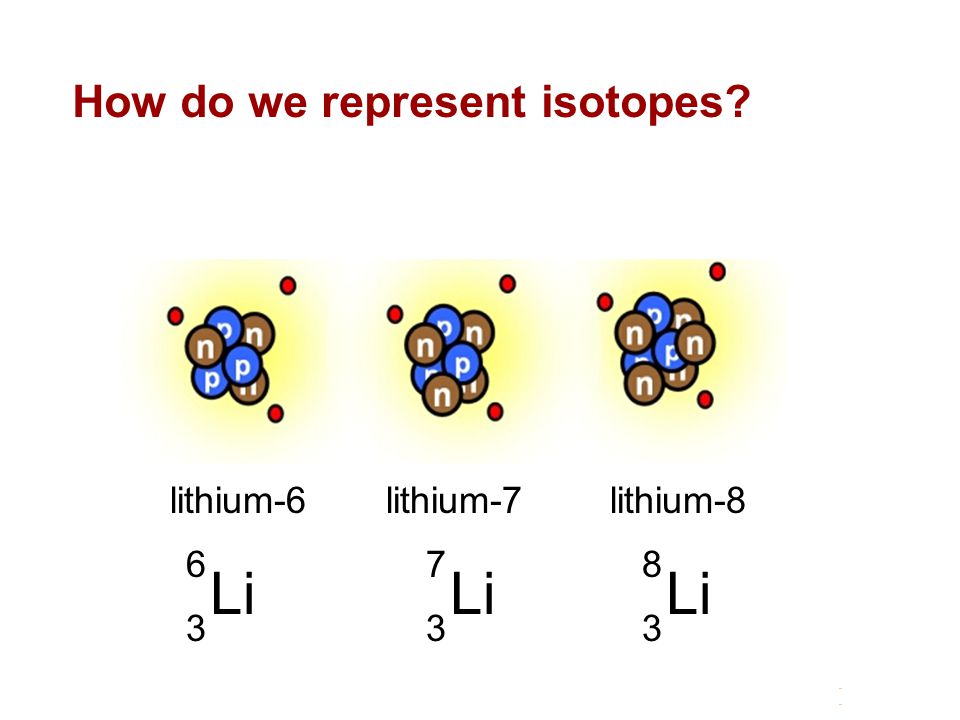
The two naturally occuring isotopes of lithium are Li-6 and Li-7. The table below shows the atomic m
60. Lithium exist in nature in the form of two isotope li 6 and li 7 with atomic masses 6.0151u and 7.0160u and the percentage 8.24 and 91.76 respectively. Calculate average atomic mass.
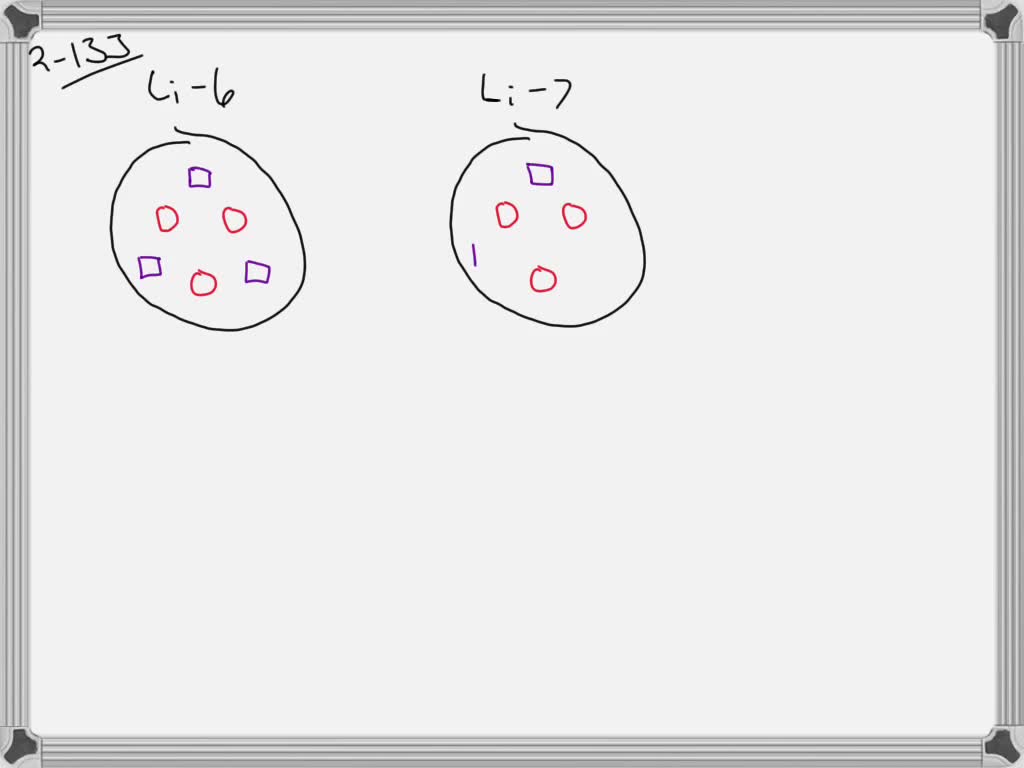
SOLVED:Lithium has two naturally occurring isotopes: Li-6 (natural abundance 7.5% ) and Li-7 (natural abundance 92.5% ). Using circles to represent protons and squares to represent neutrons, draw the nucleus of each