Efficacy and safety of ixekizumab over 4 years of open-label treatment in a phase 2 study in chronic plaque psoriasis - Journal of the American Academy of Dermatology

Figure 3. | Safety and Efficacy of Open-label Subcutaneous Ixekizumab Treatment for 48 Weeks in a Phase II Study in Biologic-naive and TNF-IR Patients with Rheumatoid Arthritis | The Journal of Rheumatology

These highlights do not include all the information needed to use TALTZ safely and effectively. See full prescribing information for TALTZ.TALTZ ( ixekizumab) injection, for subcutaneous useInitial U.S. Approval: 2016

Fillable Online accessdata fda LABEL. TALTZ (ixekizumab) injection, Eli Lilly and Company - accessdata fda Fax Email Print - pdfFiller

Efficacy and safety of ixekizumab over 4 years of open-label treatment in a phase 2 study in chronic plaque psoriasis - ScienceDirect
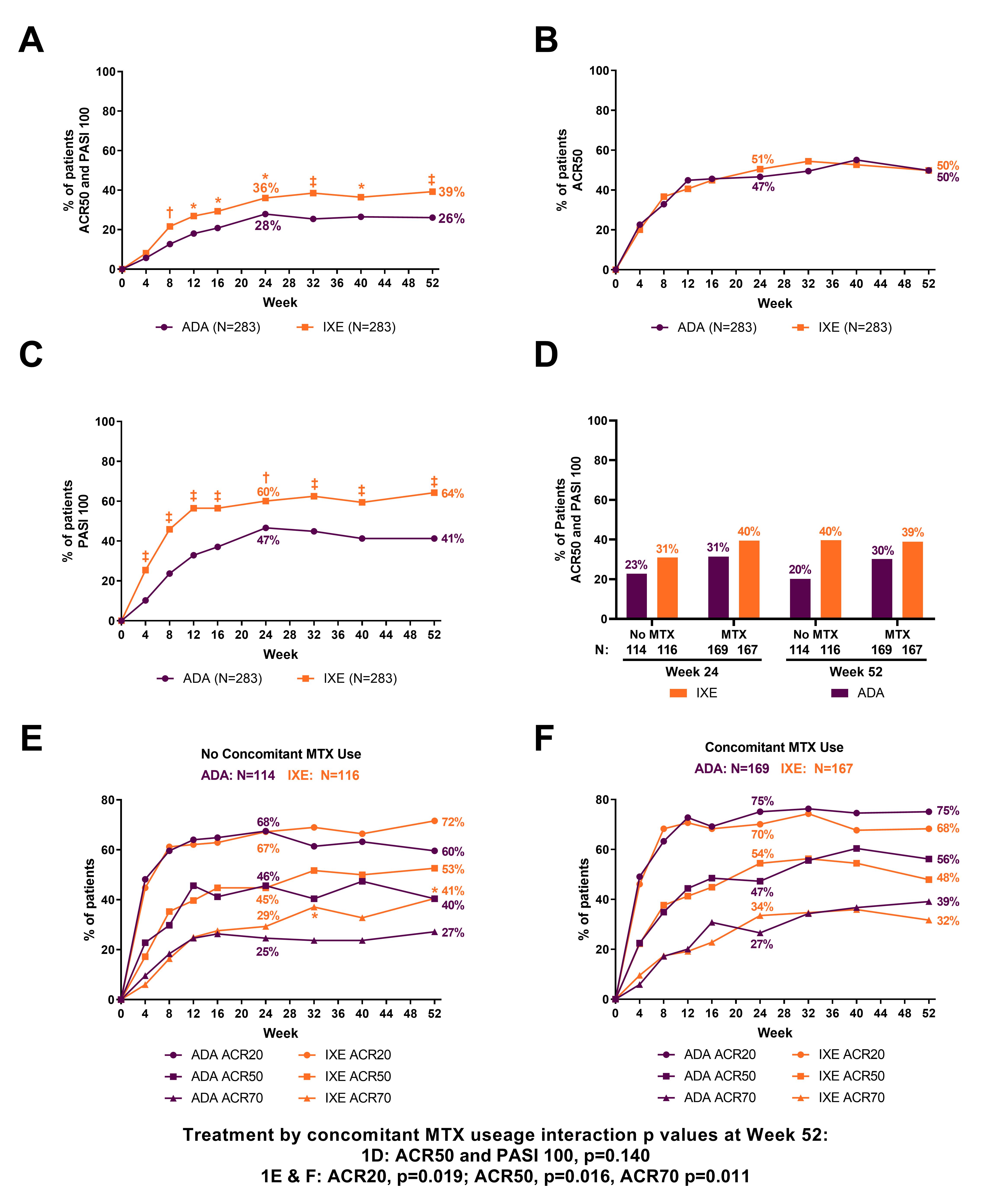
A Head-to-Head Comparison of Ixekizumab and Adalimumab in Biologic-Naïve Patients with Active Psoriatic Arthritis: Efficacy and Safety Outcomes from a Randomized, Open-Label, Blinded Assessor Study Through 52 Weeks - ACR Meeting Abstracts

Early Onset of Clinical Improvement with Ixekizumab in a Randomized, Open- label Study of Patients with Moderate-to-severe Plaque Psoriasis – JCAD | The Journal of Clinical and Aesthetic Dermatology

Long‐term efficacy and safety results from an open‐label phase III study (UNCOVER‐J) in Japanese plaque psoriasis patients: impact of treatment withdrawal and retreatment of ixekizumab - Umezawa - 2019 - Journal of

Taltz 80 mg solution for injection in pre-filled syringe - Summary of Product Characteristics (SmPC) - (emc)

LB0005 MULTICENTRE, RANDOMISED, OPEN-LABEL, ASSESSOR-BLINDED, PARALLEL-GROUP HEAD-TO-HEAD COMPARISON OF THE EFFICACY AND SAFETY OF IXEKIZUMAB VERSUS ADALIMUMAB IN PATIENTS WITH PSORIATIC ARTHRITIS NAIVE TO BIOLOGIC DISEASE-MODIFYING ANTI-RHEUMATIC ...

A 52-week, open-label study of the efficacy and safety of ixekizumab, an anti-interleukin-17A monoclonal antibody, in patients with chronic plaque psoriasis - ScienceDirect

Study design for administration of ixekizumab via a prefilled syringe... | Download Scientific Diagram

PDF) Ixekizumab treatment improves fingernail psoriasis in patients with moderate-to-severe psoriasis: Results from the randomised, controlled and open-label phases of UNCOVER-3